Abstract
Introduction
Minimal residual disease (MRD) has emerged as a key parameter in the evaluation of multiple myeloma (MM) response to therapy. Next-generation sequencing (NGS) methods detecting IGH clonal rearrangements have demonstrated clear advantages over allele-specific oligonucleotide PCR (ASO-PCR) to detect low MRD levels in MM. However, only one commercial strategy (LymphoSight™, Sequenta, San Francisco, CA) has proven a real utility in clinical practice and accordingly, has been introduced in the new response criteria of the International Myeloma Working Group (IMWG, Kumar et al, Lancet Oncol 2016). However, additional commercial or academic efforts to evaluate MRD by NGS in MM are very welcome. We have evaluated another commercial kit (LymphoTrack®, Invivoscribe, San Diego, CA) to validate its potential applicability and usefulness in the detection of MRD in MM patients, and compared their results with those obtained with multiparametric flow cytometry (MFC).
Methods
Bone marrow samples (n=220) from a cohort of 110 patients were evaluated at two time-points: diagnosis and at the post-treatment response achievement (median, 3 months after transplant, n=89; or at the end of therapy, n=21). All patients had been enrolled in clinical trials from the Spanish MM group: GEM2000 MM (n=21), GEM2010 (n=20) and GEM2012 (n=69). We had previously identified tumor clonal VDJH rearrangements in diagnostic samples through the amplification of IGH-FR1 regions followed by conventional Sanger sequencing. We then assessed MRD levels in follow-up samples using the LymphoTrack® IGH FR1 Assay Panel. More than 650 ng DNA (~105 cells) per reaction were used in order to warrant a sensitivity of 10-5 or better. 1 μL spike-in DNA corresponding to 1000 clonal cells was added to the reaction to allow the absolute quantification of tumor plasma cells. Amplicons were then sequenced in a MiSeq® platform (Illumina, San Diego, CA), processed using the LymphoTrack® software, and results were compared with those obtained by MFC.
Results
Myeloma-specific clonal rearrangements and CDR3 regions were obtained in all 110 patients. Therefore, we tested MRD in their corresponding follow-up samples. Five samples (4.5%) were excluded from the final MRD analysis due to low read counts in the post-therapy sample. Accordingly, 105 cases were valid for MRD analysis: 59 were positive and 46 were negative. A high correlation between MFC and NGS MRD results was observed (R=0.934) although we found 20 discordant cases: eight cases were negative by MFC but positive by NGS, and 12 cases were positive by MFC but negative by NGS.
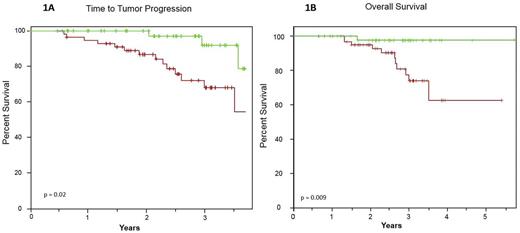
The Time to Tumor Progression (TTP) was significantly longer in the MRD- than in the MRD+ subsets by NGS, providing a projected 3-year time to tumor progression of 92.0% vs. 68.1%, respectively (p=0.02). This was translated into a better Overall Survival for patients with a negative MRD, who had a 5-year projected OS of 97.4% compared with 59% for patients with a positive MRD (p=0.009) (Figures 1A and 1B).
Among the 18 patients who experienced progression, 13 were MRD positive by MFC and NGS, 2 were MRD negative by MFC and NGS. There were only 3 cases with discordant results: two of them were MFC+ but NGS-, while the other one was MFC- but NGS+.
Conclusions
The applicability of the Lymphotrack® was very high in this study (95.5%), similar to that reported in other studies for MFC and different NGS approaches. There was a high correlation between MRD levels by sequencing and MFC, with an excellent capacity to predict the outcome. These results reinforce the usefulness of the MRD assessment by NGS for patient risk stratification in MM and provide a new approach easily available for most laboratories. This study could help to spread the NGS methodology for MRD assessment in MM and to apply the new standardized response criteria recently approved by the IMWG.
Paiva: Amgen: Honoraria; Sanofi: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria; Novartis: Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; EngMab: Research Funding. Rosinol: Celgene: Honoraria; Janssen: Honoraria. Ocio: Novartis: Consultancy, Honoraria; Celgene: Honoraria, Research Funding; Mundipharma: Research Funding; Array Pharmaceuticals: Research Funding; Takeda: Consultancy, Honoraria; AbbVie: Consultancy; Pharmamar: Consultancy; Seattle Genetics: Consultancy; Amgen: Honoraria, Research Funding; BMS: Honoraria; Janssen: Honoraria; MSD: Research Funding. Oriol: Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia; Celgene: Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau. Mateos: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lahuerta: Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria. San Miguel: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal